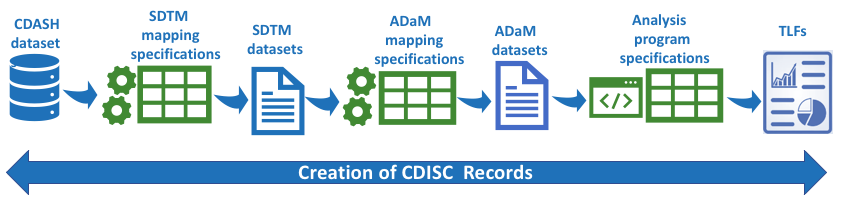
Clinical Data Interchange Standards Consortium (CDISC) has provided extensive guidelines for the collection, analysis and submission of clinical trial data for regulatory compliance purpose. Submitting data compliant with CDISC is mandatory for new drug development.
We skilfully employ all CDISC standards like SDTM, ADaM, CDASH and remain current with FDA submission. We provide best practice solution that include advice on compliance, selecting the right studies for legacy conversion and efficient end to end delivery that could reduce time and cost in overall study. Our CDISC standard specialist use the latest technology framework to ensure compliance of standards.
Our services includes :
- Developing company standards based on CDISC current models
- Optimise data flow from data capture CDASH to SDTM to ADaM to TLFs
- Conversion of Legacy data to SDTM datasets
- Compliant Submission Data Packages -SDTM annotated CRF, define.xml
- Analysis ready ADaM domains, ADaM define.xml
- Post submission support
- SDTM / ADaM Compliant integrated datasets

